

Affiliations
ABSTRACT
Midostaurin is a FMS-like tyrosine kinase 3 (FLT3) inhibitor used in FLT3-positive acute myeloid leukaemia (AML). Midostaurin has been rarely associated with Sweet’s syndrome. We present a case of a female who developed Sweet’s syndrome due to midostaurin. After skin biopsy was taken from the rash, methylprednisolone treatment was started. On the 15th day of the treatment, the patient’s rash and bilateral pretibial oedema regressed. Sweet’s syndrome is characterised by skin rash that consists of tender erythematous nodules, papules or plaques and also fever. To the best of our knowledge, there are four case reports of Sweet’s syndrome due to midostaurin. In the present case, the rash had developed on the 17th day of midostaurin treatment, which is different from these other case reports. We aimed to draw attention to Sweet’s syndrome, which is a serious side effect of midostaurin.
Key Words: Leukaemia, Sweet’s syndrome, Erythema, Skin.
INTRODUCTION
The most common type of acute leukaemia in adults is acute myeloid leukaemia (AML). FMS-like tyrosine kinase 3 (FLT3) mutation is the most common genomic alteration in AML, accounting for 1/3 of newly diagnosed adult patients.1 FLT3 mutations are subdivided into internal tandem duplication (ITD) and point mutations in the tyrosine kinase domain (TKD). These mutations cause ligand-independent FLT3 signalling and cellular proliferation. These mutations are indicative of poor prognosis. There are treatment options such as midostaurin, gilteritinib and sorafenib, which are FLT3 inhibitors.
Sweet’s syndrome is an uncommon inflammatory disease. It presents with fever, leukocytosis, and dermal neutrophilic lesions. Midostaurin may rarely cause this syndrome. Here, we present a case of Sweet’s syndrome associated with midostaurin.
CASE REPORT
A 46-year female was admitted in the emergency department with fever along with shivering and weakness. There were no signs of any infection. White blood cell (WBC) count was 2.79×109/l, neutrophil (Neu) count was 1.03×109/l, haemoglobin (Hb) was 9 g/dl, and platelets (Plt) were 149×109/l at the time of admission.
Ferritin, vitamin B12, and folate values were normal. No atypical cells/evidence of dysplasia were observed in peripheral blood smear. Fluorescent labelled aerolysin (FLAER) test for paroxysmal nocturnal haemoglobinuria (PNH) was negative.
No organomegaly was found on abdominal ultrasound. There was no lymphadenopathy or evidence of malignancy in neck-thorax-abdomen computed tomography (CT). Bone marrow biopsy was non-diagnostic, and there were no signs of dysplasia. As fever, sweating, and bicytopenia continued after 2 months, bone marrow aspiration-biopsy and flow cytometric examination were performed for the differential diagnosis of infectious events including mycobacterium tuberculosis and acute leukaemia. Up to 36% of cells with scanty cytoplasm, large, homogeneous nuclei, and blastic morphology were observed in bone marrow aspiration. Also, flow cytometry and bone marrow biopsy results were compatible with AML.
We started 3+7 chemotherapy protocol. Piperacillin-tazobac-tam was started on the 5th day of the treatment due to the development of febrile neutropenia. Piperacillin-tazobactam treatment was stopped after 14 days because of the achievement of clinical response. Midostaurin 2×50 mg/day was added to the treatment after FLT3-TKD resulted as 45% positive. On the 17th day of midostaurin treatment, erythematous, maculopapular rash, and bilateral pretibial oedema appeared on the both lower extremities of the patient (Figure 1). Skin biopsy was taken from the rash and 1 mg/kg/day methylprednisolone treatment was started. Cryoglobulin, antinuclear antibody (ANA) test, p-ANCA and c-ANCA were negative. On the 15th day of methylprednisolone treatment, a marked reduction in the patient’s rash and bilateral pretibial oedema were observed (Figure 2). Dense neutrophilic infiltrate in reticular dermis, perivascular neutro-phils, and lymphocyte infiltration, and erythrocyte extravasation were observed on skin biopsy. The patient was diagnosed as midostaurin-induced Sweet’s syndrome. Bone marrow aspiration and biopsy results confirmed remission after remission induction therapy.
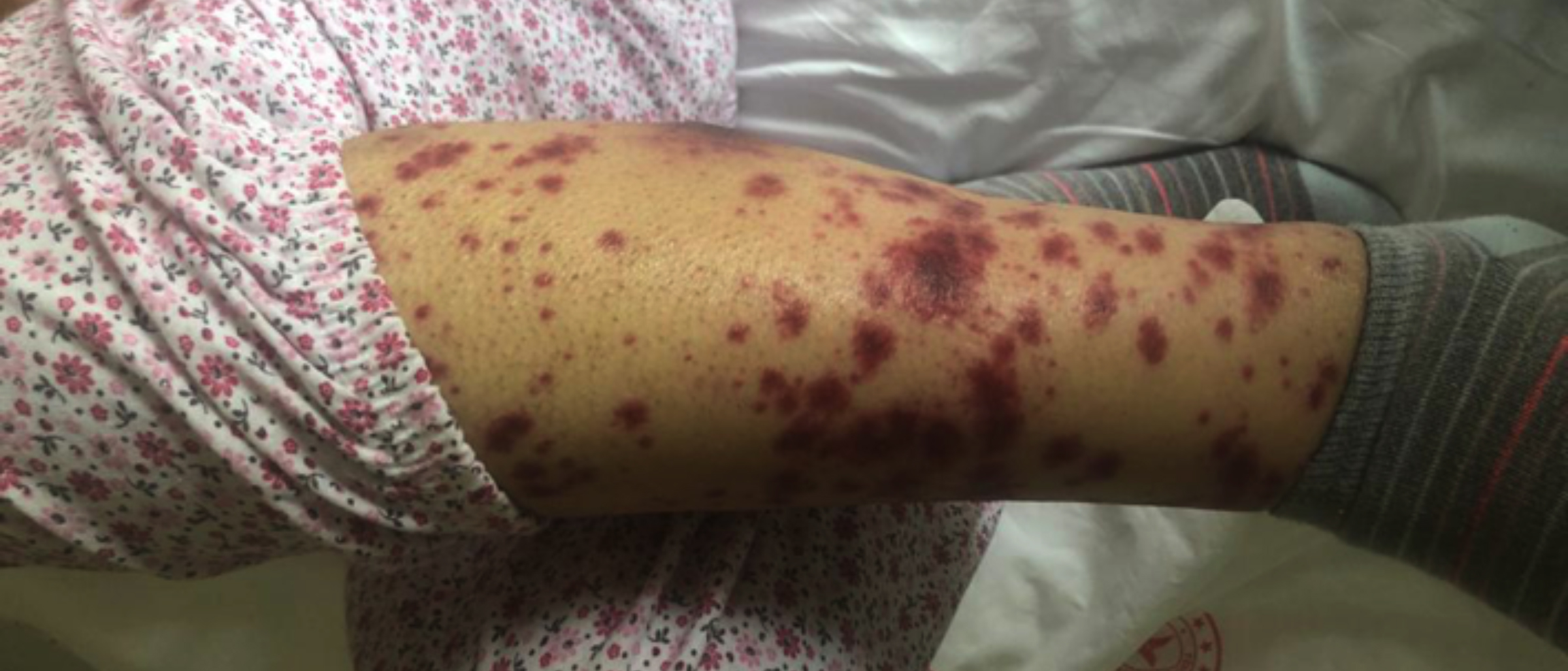 Figure 1: On the 17th day of midostaurin treatment, erythematous, maculopapular rash, and bilateral pretibial oedema appeared on both the lower extremities of the patient.
Figure 1: On the 17th day of midostaurin treatment, erythematous, maculopapular rash, and bilateral pretibial oedema appeared on both the lower extremities of the patient.
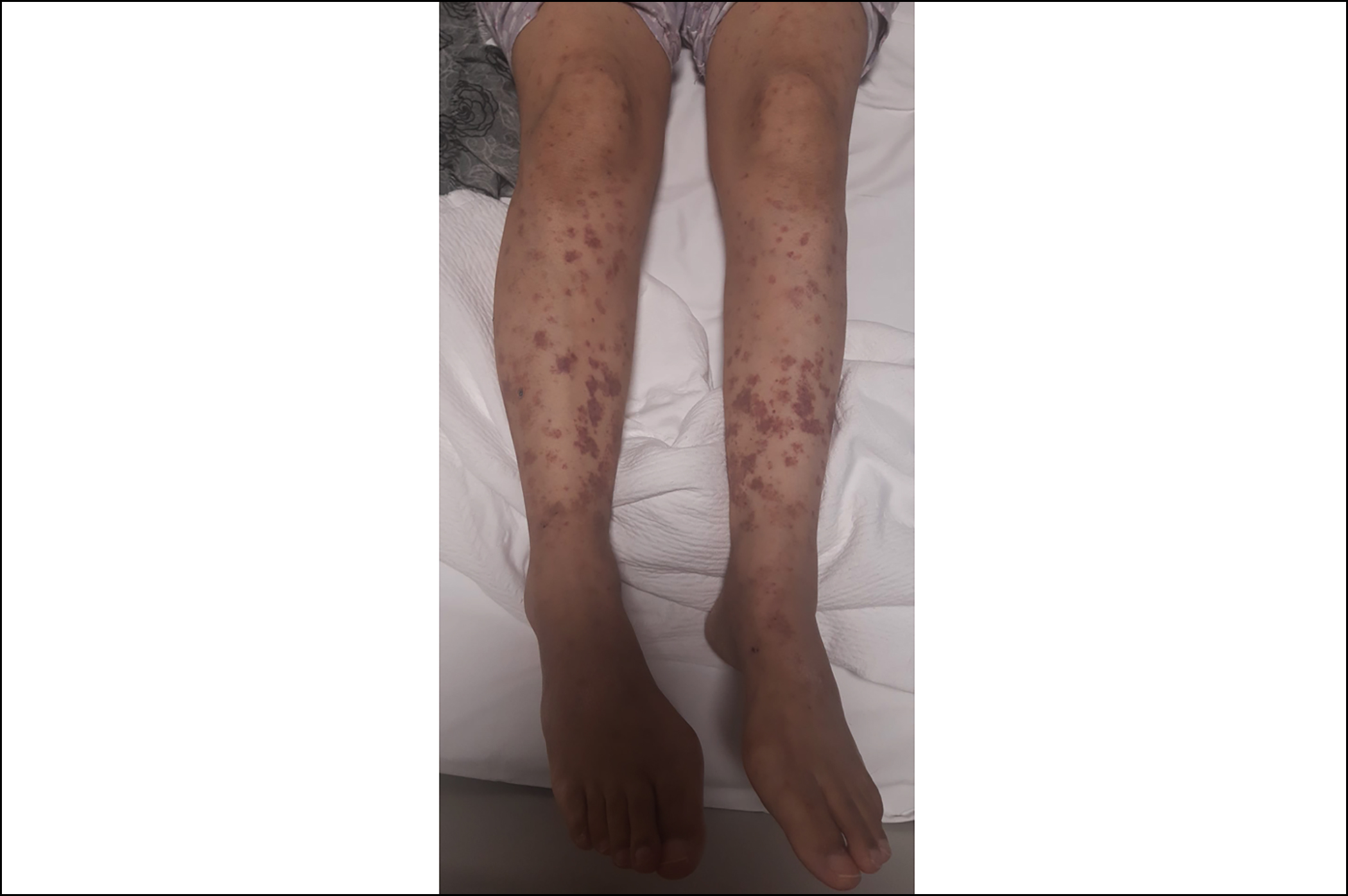 Figure 2: On the 15th day of methylprednisolone treatment, a marked reduction in the patient’s rash, and bilateral pretibial oedema was observed.
Figure 2: On the 15th day of methylprednisolone treatment, a marked reduction in the patient’s rash, and bilateral pretibial oedema was observed.
DISCUSSION
Sweet’s syndrome is characterised by skin rash that consists of tender erythematous nodules, papules or plaques and fever, divided into three subtypes: drug-induced Sweet’s syndrome, classical Sweet’s syndrome, and malignancy-associated Sweet’s syndrome.
Classical Sweet’s syndrome is the most common subtype. It is mostly associated with infections (especially gastrointestinal and upper respiratory tract infections), inflammatory bowel disease, and pregnancy.2
Up to 15-20% of patients have underlying neoplasms, and 85% of these cases have haematological malignancies.3 Lesions may appear before, concomitant or after the cancer diagnosis. AML is the most common malignancy associated with malignancy-associated Sweet syndrome. Kazmi et al. found that the deletion of chromosome 5 or 5q karyotype, FLT3 mutations and AML with myelodisplasia-related features were more common in patients with Sweet’s syndrome.4
The potential causes of drug-induced Sweet’s syndrome are granulocyte colony stimulating factor (G-CSF), azathioprine, furosemide, hydralazine, trimethoprim-sulfamethoxazole, mino-cycline, and oral contraceptives.2 Sweet’s syndrome often occurs two weeks after taking the drug in patients who did not have any exposure to the inciting drug before.5
The most common extracutaneous symptom is fever, that may precede skin lesions by days to weeks. Cutaneous manifestations are generally painful, red to purple coloured papules or nodules or plaques. The cutaneous eruption distributes asymmetrically and is mostly seen in the upper extremities.2
Drug-induced Sweet’s syndrome can improve after stopping the therapy with the offending drug and treatment of underlying malignancy can result in improvement.2
A treatment with corticosteroids results in adequate response. Prednisone can be given at a dose of 0.5-1 mg/kg/day. The symptoms generally start to resolve in 48 hours. The dermatologic lesions often resolve within one to two weeks. When disease control is achieved, prednisone dose can be tapered and discontinued over the course of four to six weeks. If corticosteroid treatment fails, potassium iodide, colchicine, dapsone, cyclosporine, naproxen, indomethacin, clofazimine, and α-interferon can be used.6
To the best of our knowledge, four case reports about Sweet’s syndrome due to midostaurin have been reported in the literature.7-10 The presence of fever and erythematous maculopapular rash in the present case, neutrophilic infiltration on skin biopsy, the onset of rashes after midostaurin, the absence of active leukaemia (proven by bone marrow biopsy), secondary malignancy, no active infection findings and the dramatic response of the patient to systemic steroids, all support midostaurin-induced Sweet’s syndrome. Contrary to the case report of Yasin et al.10 this case is different because of the rash developed on the 17th day of midostaurin treatment.
The use of targeted agents, such as midostaurin, is becoming more common in FLT3-positive AML. Although midostaurin is an effective drug against FLT3-mutated AML, it has side-effects. It should be noted that midostaurin may rarely cause Sweet’s syndrome.
PATIENT’S CONSENT:
Informed consent was obtained from the patient to publish the case.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MGT: Conceived the design, did analysis and interpretation of data, and drafting.
MOY: Designed and drafed the study.
All authors approved the final version of the manuscript to be published.
REFERENCES